Periodic Table of Elements Worksheets
About These 15 Worksheets
The Periodic Table is often seen as a complex and intimidating chart, an intricate arrangement of symbols and numbers that many students feel is impossible to fully grasp. However, with the right approach and tools, the Periodic Table transforms from a daunting maze into a clear map of knowledge. That’s where these carefully designed worksheets come in. They are not just simple exercises-they are your gateway to mastering one of the most important tools in chemistry. These worksheets are your personal training ground, crafted to help you dive deep into the table’s structure, unlock its secrets, and make sense of the elements it contains.
Understanding the Periodic Table is more than just memorizing rows and columns of elements. It’s about recognizing patterns, discovering relationships between elements, and appreciating the logic behind the table’s organization. Each element on the table tells a story: a story of its properties, its behavior in reactions, and its role in the natural world. The worksheets are designed to make these stories accessible and engaging. By working through them, you will not only sharpen your ability to recall element names and symbols but also learn how to interpret the key information the table provides.
As you practice, you’ll begin to see the Periodic Table as more than a collection of facts. It will become a powerful tool in your hands-one that you can use to predict trends, identify similarities between elements, and even foresee how different elements will react with each other. These worksheets are structured to guide you through this learning process, offering both challenges and opportunities to apply your knowledge in meaningful ways.
At first, locating specific elements or understanding their properties may feel like a slow and difficult process. You might spend time looking up symbols or double-checking atomic numbers, but that’s okay. Every moment spent interacting with the table is a step towards mastery. As with learning any new skill, practice is essential. These worksheets are designed to give you ample opportunity to practice until navigating the Periodic Table becomes second nature. Over time, what once seemed unfamiliar will become as comfortable as reading a book.
Imagine being able to glance at an element and immediately understand not only its basic characteristics but also how it fits into the bigger picture of chemistry. Picture yourself quickly identifying trends, such as how atomic radius increases or decreases as you move across or down the table, or predicting the types of bonds an element is likely to form. This level of fluency may seem distant now, but with consistent effort and the help of these worksheets, it is entirely within your reach.
The Periodic Table is a dynamic and versatile tool, constantly evolving as new elements are discovered and added. By using these worksheets, you’ll not only build a solid foundation of knowledge but also cultivate the skills needed to keep up with these changes. You’ll learn to approach the table with confidence, understanding how each element fits into the broader context of chemistry. These worksheets are more than a study tool-they are an investment in your long-term understanding of science.
What sets these worksheets apart is their focus on active engagement. You’re not passively reviewing information but actively participating in your own learning. Each exercise is designed to challenge you, encouraging you to think critically about the relationships between elements and to apply your knowledge to solve problems. The more you engage with the material, the more you’ll internalize the patterns and trends that are so essential to understanding chemistry.
Types of Problems
Element Identification – These problems might ask you to identify an element based on its symbol or name. For instance, you might be given the symbol “O” and asked to write its name, which is “Oxygen”. Or, you might see a question like, “Which element has the symbol ‘Au’?” The answer is “Gold”.
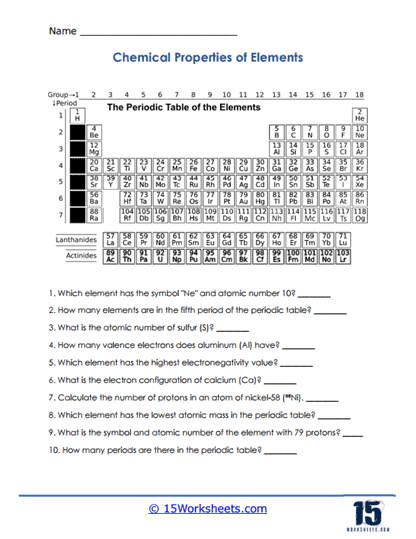
Element Properties – Once you know an element’s name and symbol, you’ll want to learn about its properties. Questions might ask, “Is Chlorine a metal or non-metal?” or “What state of matter is Helium at room temperature?” By answering, you’ll get familiar with how elements behave and their different traits.
Element Location – The Periodic Table is organized in a very specific way. Some problems might ask you to find where an element is located. For example, “Which period (row) is Lithium in?” or “In which group (column) will you find Neon?” Knowing the position helps in understanding the element’s behavior.
Atomic Number and Atomic Mass – Each element on the Periodic Table has an atomic number (how many protons it has) and an atomic mass (roughly, the total weight of its protons and neutrons). Problems might ask, “What’s the atomic number of Carbon?” or “What’s the atomic mass of Iron?” These numbers give clues about the element’s structure and relationships with others.
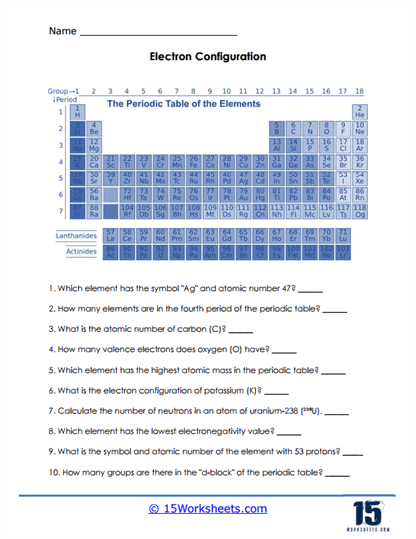
Electron Configuration – This might sound a bit complicated, but it’s all about understanding how many electrons are around an atom and where they are. Questions could include – “How many electron shells does Sodium have?” or “How many electrons are in the outermost shell of Oxygen?” Knowing this helps in understanding why some elements react with others.
Element Families or Groups – Elements in the same column or group usually have similar properties. Questions might ask, “Which elements are Noble Gases?” or “Name an element in the Alkaline Earth Metals group.” Recognizing these families helps in predicting how an element might behave.
Comparing Elements – Sometimes, you’ll be asked to compare two or more elements. For example, “Which is heavier, a molecule of Oxygen or Nitrogen?” or “Which element is more reactive, Potassium or Lithium?” Comparing helps you see the relationships between different elements.
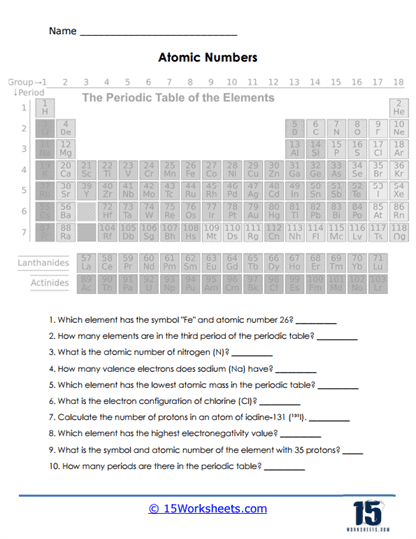
What is the Periodic Table of Elements?
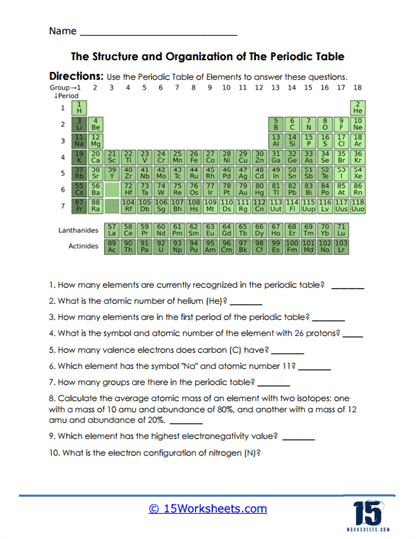
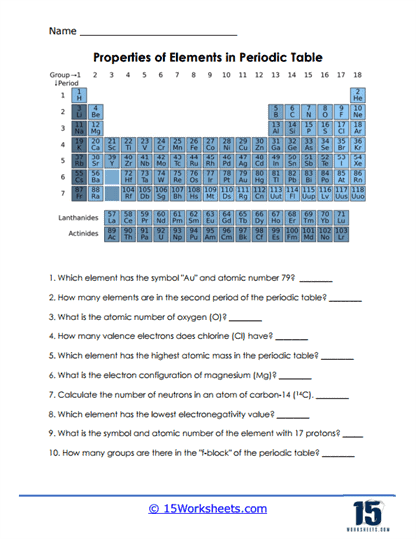
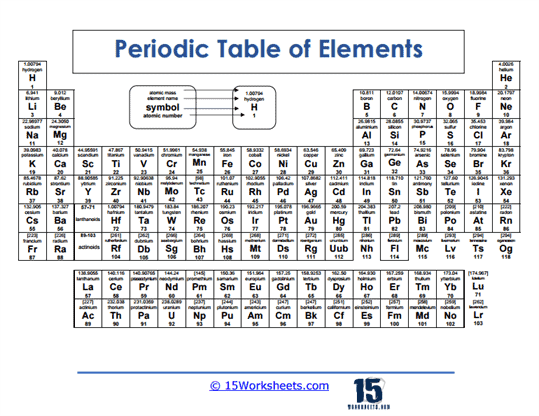
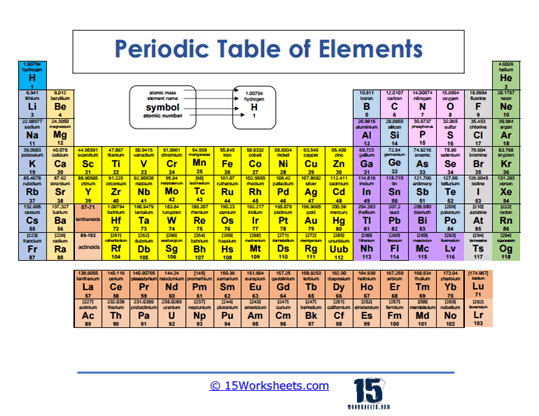
The Periodic Table of Elements is a scientific chart that organizes all known chemical elements in a structured manner based on their properties. It was first developed by Russian chemist Dmitri Mendeleev in 1869, who arranged elements according to their atomic mass and observed that certain properties recurred periodically. This early version of the table has since evolved, and modern versions are organized by increasing atomic number, which corresponds to the number of protons in an element’s nucleus. The periodic table is a fundamental tool in chemistry, physics, and many other scientific fields, providing an easy reference to important information about elements, including their atomic structure, chemical behavior, and relationships with other elements.
The periodic table is arranged in rows, called periods, and columns, known as groups or families. Each period corresponds to a different energy level of electrons surrounding an atom’s nucleus. As you move from left to right across a period, the atomic number of the elements increases, meaning that each subsequent element has one more proton and one more electron than the previous element. While moving across a period, the chemical properties of the elements change progressively because of changes in electron configuration. On the other hand, elements within the same group (column) have similar chemical and physical properties because they have the same number of electrons in their outermost energy level, or valence shell. These outer electrons largely determine how an element reacts with others.
For example, Group 1 of the periodic table contains elements known as the alkali metals, like lithium (Li), sodium (Na), and potassium (K). These elements all have a single electron in their outer shell, making them highly reactive and prone to losing that electron in chemical reactions to form positive ions. Group 18, on the other hand, contains the noble gases, such as helium (He), neon (Ne), and argon (Ar). These elements have full outer electron shells, making them very stable and chemically inert under most conditions. This organization of elements into groups with shared characteristics makes it easier to predict the behavior of unknown elements or to understand the chemical behavior of compounds.
The periodic table is a powerful tool because it condenses a vast amount of information into a simple visual format. By looking at the table, one can quickly gather key facts about an element, such as its atomic number, atomic mass, and even its general chemical reactivity. In addition, the table reveals trends in properties, such as electronegativity, ionization energy, and atomic radius. For instance, elements become more electronegative as you move from left to right across a period, and they tend to become larger as you move down a group. These trends help scientists and students predict how elements will interact in chemical reactions, as well as explain the structure and properties of complex compounds.