Atoms, Elements, Compounds Worksheets
About These 15 Worksheets
Atoms, elements, and compounds form the foundational concepts in the study of chemistry. Teaching these basic units of matter and how they interact is crucial for students to grasp more complex chemical theories and reactions. Atoms, elements, and compounds worksheets serve as instrumental tools for educators to assess and reinforce a student’s understanding of these fundamental topics. These worksheets contain a variety of exercises designed to help students grasp these concepts at a deeper level.
Types of Exercises on These Worksheets
Multiple Choice Questions (MCQs) – These assess a student’s basic knowledge about the concepts.
Example: Which of the following is not an element? (A) Gold (B) Water (C) Oxygen (D) Neon.
Atoms of the same element but with different numbers of neutrons are called? (A) Isomers (B) Isotopes (C) Ions (D) Molecules.
True or False – These can test students’ foundational knowledge and dispel misconceptions.
Example: All atoms of an element are identical. (True/False)
Fill in the Blanks – These types of questions force students to recall terms or concepts without the aid of multiple choices.
Example: A _______ is the smallest unit of an element that maintains the properties of that element.
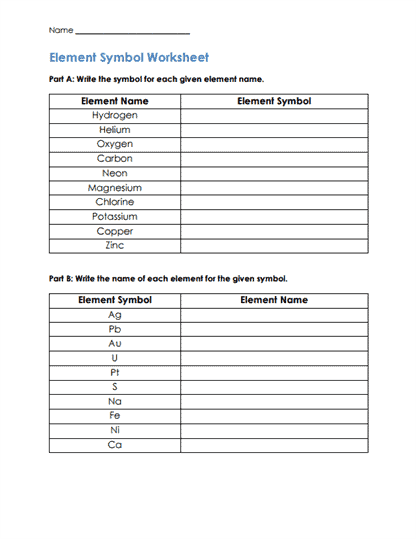
Matching – Often used to connect symbols with their corresponding elements or descriptions with terms. Students might have to match symbols like “H”, “O”, “Na” with “Hydrogen”, “Oxygen”, “Sodium”.
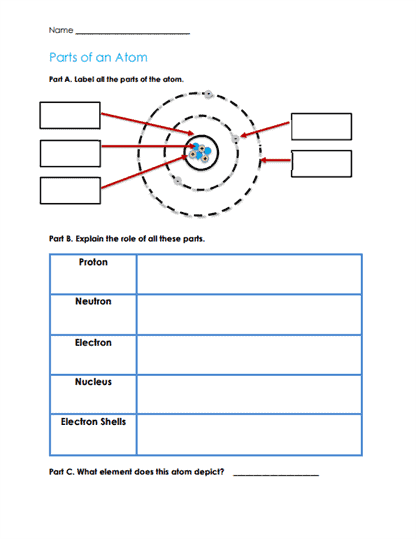
Diagram Labeling – Visual aids where students label parts of an atom (e.g., electrons, nucleus, protons, neutrons) or differentiate between diagrams of individual atoms, molecules of elements, and compounds.
Composition and Decomposition – Exercises where students identify which elements compose a given compound or vice versa.
Example: Given water, students should be able to identify that it is composed of Hydrogen and Oxygen.
Balancing Chemical Equations – Though a bit more advanced, some worksheets might introduce simple chemical reactions, asking students to balance them, ensuring conservation of atoms.
Short Answer Questions – These probe deeper understanding.
Example: Explain the difference between an element and a compound. or Describe the structure of an atom.
Concept Maps – Students might be asked to create a concept map linking terms and concepts related to atoms, elements, and compounds, demonstrating their interconnected nature.
Atoms, elements, and compounds worksheets are indispensable tools in the realm of learning chemistry. They offer educators a structured method to evaluate and bolster a student’s grasp of these pivotal concepts. The array of exercises-ranging from multiple-choice questions to group discussions-ensures a holistic approach to learning. This multifaceted teaching strategy caters to diverse learning styles, making the intricate world of atoms, elements, and compounds more accessible and engaging to students.
What Are Atoms?
Atoms are the basic units of matter and the fundamental building blocks of the universe. They are incredibly tiny particles from which every element is made. The concept of the atom has existed for thousands of years, but our understanding of them has changed significantly over time as science has advanced.
Components of an Atom
Atoms are composed of a central nucleus and a cloud of electrons that orbit the nucleus. The nucleus itself contains two types of particles – protons and neutrons.
Protons – Positively charged particles found in the nucleus of an atom. The number of protons in an atom determines the element to which it belongs. For example, a hydrogen atom has one proton, while a carbon atom has six.
Neutrons – Neutral particles, meaning they have no charge. They also reside in the nucleus. Neutrons contribute to the mass of the atom but don’t influence the atom’s chemical identity.
Electrons – Negatively charged particles that orbit the nucleus in specific regions called electron shells or orbitals. Electrons play a crucial role in chemical reactions and in the formation of chemical bonds between atoms. The number of electrons in a neutral atom equals the number of protons.
Atoms of the same element (i.e., with the same number of protons) can have different numbers of neutrons. Such variants are called isotopes. For instance, carbon-12 and carbon-14 are both carbon atoms, but carbon-12 has 6 neutrons while carbon-14 has 8. Isotopes can have different physical properties, and some can be radioactive.
What Are Elements?
An element is a pure substance that cannot be broken down into simpler substances by chemical means. The concept of an element is foundational in chemistry, as elements serve as the building blocks for all forms of matter we encounter in our universe.
Unlike compounds, which are composed of two or more types of atoms chemically bonded together, an element is made up of only one type of atom.
Every element is defined by its atomic number, which is the number of protons in the nucleus of its atoms. This atomic number determines the element’s chemical identity. For example, all atoms with 1 proton are hydrogen, while all atoms with 2 protons are helium.
What Are Compounds?
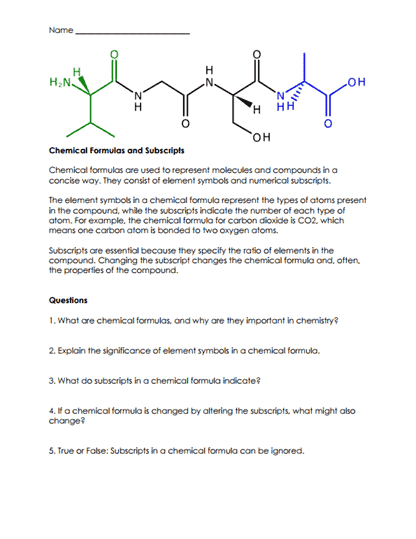
The vast majority of substances we encounter daily are compounds, from the water we drink to the air we breathe (which contains oxygen O2, nitrogen N2, carbon dioxide CO2, and more). The ability of elements to combine and form compounds results in the incredible diversity of matter we observe in our world. The study of compounds allows scientists to predict reactions, create new materials, and understand the molecular intricacies of life itself.
The formation of a compound involves the creation of chemical bonds between atoms. These bonds can be covalent (where atoms share electrons), ionic (where atoms transfer electrons from one to another), metallic, or even hydrogen bonds.
Elements in compounds always combine in a fixed ratio by mass. For instance, water (H2O) is a compound consisting of hydrogen and oxygen, always in a 2:1 ratio by atom count.
Distinct Properties
A hallmark of compounds is that they exhibit properties entirely different from the elements that compose them. For instance:
Sodium (Na) is a soft, highly reactive metal.
Chlorine (Cl) is a yellow-green, toxic gas.
When combined, they form sodium chloride (NaCl), or table salt, which is a white, crystalline substance essential for human consumption.