Acids and Bases Worksheets
About These 15 Worksheets
These worksheets will help students better understand the nature of solutions that are acidic or basic. Students will learn the relative strength that a solution exhibits and what properties would most likely be associated with it based on that characteristic.
In the vast universe of chemistry, the concepts of acids and bases stand out as fundamental building blocks that underpin many essential chemical reactions and processes. Our collection of 15 meticulously designed worksheets delves deep into this pivotal topic, offering students a comprehensive overview, practical exercises, and real-world applications of the principles surrounding acids and bases.
Acids and bases are ubiquitous in the world around us. From the citric acid in our morning orange juice to the basic solutions used in household cleaning agents, these substances impact our daily lives. By understanding their properties, students gain a foundation that is essential for advanced chemistry studies and can appreciate the nuances of many everyday chemical reactions.
What are Acids and Bases?
Acids
Think of lemon juice. If you’ve ever tasted it, you’ll know it’s super sour. This sour taste comes from the fact that lemon juice is an acid. Acids can be found in many everyday items, like vinegar, oranges, and even soda!
An acid is a substance that can donate a proton (which is a hydrogen ion, H+) to another substance. When an acid dissolves in water, it produces H+ ions. Here are some characteristics and examples of acids:
Taste – Acids generally taste sour. Think of how citrus fruits like lemons and oranges taste.
Touch – Some acids can feel sticky to the touch.
Reactivity – Acids can react with metals to produce hydrogen gas.
pH – Acids have a pH less than 7.
Indicator Change – If you use a universal indicator or litmus paper, acids will turn blue litmus paper red.
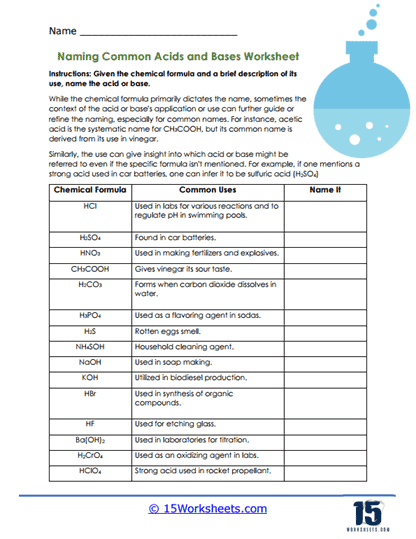
Common Examples – Citric acid (in citrus fruits), hydrochloric acid (in our stomachs), sulfuric acid (in batteries), and acetic acid (in vinegar).
Bases
On the other hand, think of soap. We don’t eat it, of course, but if you’ve ever had a tiny bit in your mouth by mistake, it tastes bitter. This is because most soaps are bases. Other examples of bases include baking soda and bleach.
A base is a substance that can accept a proton (H+) or, equivalently, produce a hydroxide ion (OH-) when dissolved in water. Here are some characteristics and examples of bases:
Taste – Bases taste bitter. But remember, it’s not safe to taste all substances to determine if they are acids or bases!
Touch – Many bases feel slippery or soapy.
pH – Bases have a pH greater than 7.
Indicator Change – In the presence of a universal indicator or litmus paper, bases will turn red litmus paper blue.
Common Examples – Sodium hydroxide (used in soap-making), ammonia (a common household cleaner), and calcium hydroxide (used in cement).
When acids and bases combine, they can neutralize each other, producing water and a salt. This is called a neutralization reaction. For example, when hydrochloric acid (an acid) reacts with sodium hydroxide (a base), they produce water and sodium chloride (common table salt).
At their core, acids are substances that can give off H+ ions, and bases are substances that can accept H+ ions or give off OH- ions. They play crucial roles in various chemical reactions and processes, both in nature and in man-made environments.
Types of Problems on Acid and Base Worksheets
Identifying Acids and Bases
One of the simplest types of questions is where you’ll be given a list of substances, and you have to identify which ones are acids and which ones are bases. For example, the worksheet might list “lemon juice, soap, vinegar, sugar”. You would then mark lemon juice and vinegar as acids and soap as a base. This helps you recognize common household items that are acids or bases.
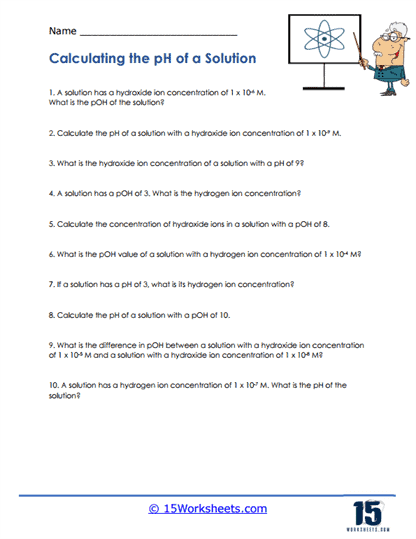
pH Scale
The pH scale is a way to measure how acidic or basic a solution is. It goes from 0 to 14, with 7 being neutral (like water). Anything below 7 is acidic, and anything above 7 is basic. A problem might ask – “If a solution has a pH of 3, is it an acid or a base?” You would then answer that it’s an acid because it’s below 7. This type of question helps you understand the pH scale and how it relates to acidity and basicity.
Neutralization Reactions
As mentioned before, when an acid and a base mix in the right amounts, they cancel each other out. This is called a neutralization reaction.
A worksheet might present a story problem like – “Sarah mixed 5 ml of acid with 10 ml of a base, but the solution was still a bit acidic. Does she need more acid or more base to make it neutral?” Using your knowledge, you’d figure out that Sarah needs more base. Such questions teach you about the magic of balancing acids and bases.
Acid and Base Strength
Not all acids and bases are created equal. Some are strong, and others are weak. A worksheet might ask – “Which is a stronger acid – lemon juice or battery acid?” The answer is battery acid. By answering such questions, you’ll get a sense of the range of strengths that different acids and bases can have.
Everyday Examples
These questions connect your learning to the real world. For instance – “If you have a bee sting (which is acidic), should you put baking soda (a base) on it? Why or why not?” The answer is yes, because a base can help neutralize the acid from the bee sting, which might help with the pain. These questions make the science of acids and bases feel more relevant and useful.