Naming Common Acids and Bases
Worksheet Description
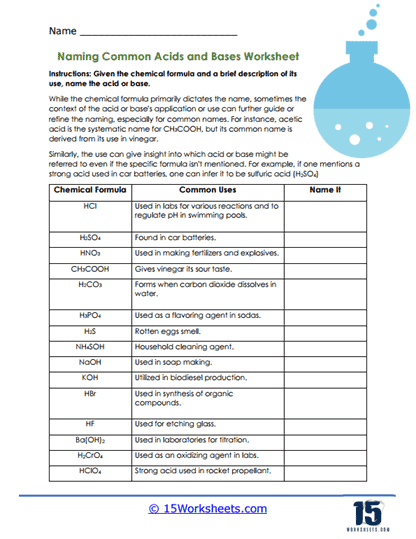
Instructions: Given the chemical formula and a brief description of its use, name the acid or base.
While the chemical formula primarily dictates the name, sometimes the context of the acid or base’s application or use can further guide or refine the naming, especially for common names. For instance, acetic acid is the systematic name for CH3COOH, but its common name is derived from its use in vinegar.
Similarly, the use can give insight into which acid or base might be referred to even if the specific formula isn’t mentioned.
Its objective is to teach or test one’s knowledge in naming various common acids and bases based on their chemical formula and the brief description of their use.
While the primary naming of acids and bases is dictated by their chemical formula, sometimes the context of the compound’s use or application can provide further guidance or confirmation. For instance, the worksheet mentions that even though CH3COOH’s systematic name is acetic acid, it’s more commonly known as vinegar.
Before starting, one should be familiar with the nomenclature of acids and bases. For acids, the prefix “hydro-” is used if the acid has only two elements. For example, HCl is named hydrochloric acid. If the acid contains oxygen, its name will often end in “-ic” or “-ous” depending on the number of oxygens.
Take cues from the provided “Common Uses” column. Some acids and bases have specific applications that can help identify them. For example, if you know that vinegar has a sour taste, you can deduce that CH3COOH is acetic acid.
